 Pharma - Freedom to Operate (Sample Report)
Pharma - Freedom to Operate (Sample Report)
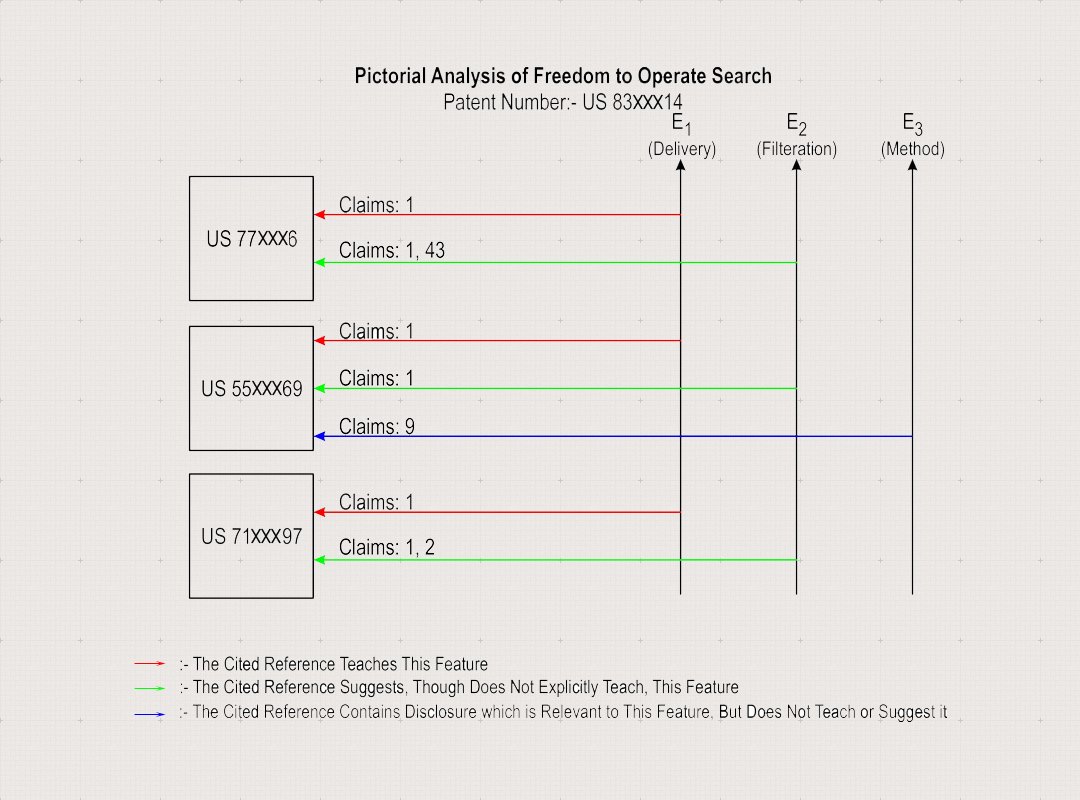
Features to Search
E1: A method of delivering a medication aerosol for treating sinusitis or rhinitis, and related neurological disorders of the cranial cavity and facial tissue.
E2: The filter comprises pores; the medication aerosol and the liquid composition do not contain a surfactant and the liquid composition comprises one or more compounds selected from the group consisting of antifungals, leukotriene antagonists, anti-TNF compounds, antihistamines, mucolytics, estrogen and progesterone. The liquid composition also comprises an antibiotic, and wherein the antibiotic is selected from the group of antibiotic classes consisting of: cephalosporins, penicillins, aminoglycocides, quinolones, tetracyclines and macrolides and also antivirals selected from the group consisting of oseltamivir, acyclovir and rimantadine. Further includes, montelukast and zafirlukast, anti-inflammatory is selected from the group consisting of budesonide, betamethasone and mometasone, mucolytic is selected from the group consisting of acetylcysteine and dornase alpha.
E3: The medication aerosol is made using a method comprising creating the medication aerosol by passing a liquid composition through a filter, and wherein the filter comprises pores, thereby forming the aerosol; and the medication aerosol and the liquid composition do not contain a surfactant.
Search Strategy
Database: AcclaimIP, USPTO, Patentscope, Espacenet, Google Patents.
Keywords:
| Set1 | Sinusitis, Rhinitis, Congestion, Bronchitis, Cranial Cavity Congestion |
| Set2 | Aerosol, Puff, Medication aerosol |
| Set3 | Antibiotics, Antifungals, Cephalosporin |
| Set4 | Surfactants, Tensile reducer |
US Classification Codes with definitions
425/43: Effervescent or Pressurized Fluid Containing
128/200.14: Liquid Medicament Atomizer or Sprayer
514/956: Gaseous or Gas Emitting Carrier or Propellant






